Work in foundries, welding, metal fabrication, and machining all requires an understanding of metal melting points. Knowing the precise temperature at which a metal changes from solid to liquid helps you choose the right materials and process conditions for any project.
What Is the Melting Point of a Metal?
The melting point is the temperature at which a metal transitions from solid to liquid form. For most pure metals, the melting and freezing temperatures are nearly identical. This point is influenced by the metal’s atomic structure, level of purity, and bonding strength.
Key Factors That Affect Melting Points
- Purity of the metal: Impurities can raise or lower melting temperatures.
- Atomic bonding: Stronger metallic bonds require more heat to separate.
- Alloy composition: Metals blended with others melt over a range instead of a single point.
- External pressure: Higher pressure can slightly change melting behavior.
- Heating rate: Rapid heating may reduce temperature accuracy during testing.
Metal Alloys vs Pure Metals
At specific temperatures, pure metals like copper and aluminum melt cleanly and consistently. In contrast, metal alloys such as steel and bronze contain multiple elements, creating a melting range instead of one fixed point. This difference occurs because alloys do not have a uniform atomic structure.
The Function of Fusion Energy
The heat of fusion is the extra energy a pure metal absorbs to melt. This energy helps the solid transform into a liquid by breaking atomic bonds. When the metal cools and solidifies again, it releases this same energy during freezing.

Melting Points of Common Metals
| Metal | Melting Point (°C) | Melting Point (°F) |
|---|---|---|
| Aluminum | 660 | 1220 |
| Brass | 930 | 1710 |
| Bronze | 950 | 1742 |
| Cast Iron | 1200 | 2192 |
| Copper | 1085 | 1985 |
| Gold | 1064 | 1947 |
| Lead | 327 | 621 |
| Nickel | 1455 | 2651 |
| Silver | 962 | 1764 |
| Steel (Carbon) | 1425–1540 | 2600–2800 |
| Tin | 232 | 449 |
| Titanium | 1668 | 3034 |
| Zinc | 420 | 788 |
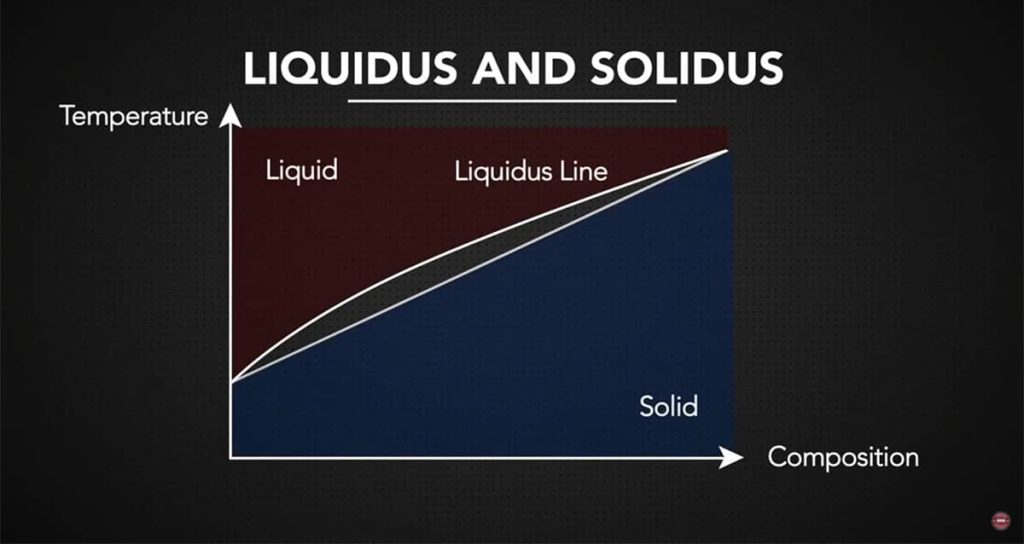
Knowing Liquidus and Solidus
For alloys, the solidus is the temperature at which melting begins, and the liquidus is the temperature where the alloy becomes fully molten. Between these two points, the metal enters a partially liquid state, often referred to as the slushy phase, where both solid and liquid phases exist together.
Test Your Knowledge on Metal Melting Points
Think you know the melting temperatures of common metals? Put your knowledge to the test with our interactive quiz on SawbladeUniversity.com. It’s a quick and engaging way to review what you’ve learned, challenge yourself, and discover new insights about metal properties and heat behavior. Head over now and see how well you understand the science behind melting points!
Why Knowing Melting Points Matters
Understanding melting points helps determine:
- Welding temperatures to prevent burn-through or distortion.
- Casting conditions for proper mold filling and flow.
- Heat treatment levels to reach specific hardness or strength.
- Material selection based on thermal resistance and use environment.
A Comparison Between Alloys and Pure Metals
Pure metals show consistent melting behavior, making them ideal for precision tasks such as electronics and jewelry. Alloys, on the other hand, offer greater durability, strength, and corrosion resistance, which makes them more practical for industrial or structural applications.
Safety and Maintenance Considerations
Always use the correct protective equipment when working with molten metals. Fire-resistant gloves, eye protection, and durable clothing are essential in high-temperature settings. Ensure proper ventilation when melting alloys that include lead, zinc, or other hazardous materials.
Choosing the Right Metal for Your Project
When selecting materials, consider melting temperature, strength, corrosion resistance, and workability. Aluminum and brass are great for general fabrication, while stainless steel and titanium perform better in high-heat or high-stress conditions.
Practical Applications of Melting Point Knowledge
- Foundry operations: Melting and casting parts to accurate temperatures.
- Welding and brazing: Selecting compatible filler metals.
- Recycling: Sorting and processing metals efficiently by melting range.
- Manufacturing: Managing heat treatment and forming processes.
Understanding Cobalt Alloy Performance
For engineers and metalworkers interested in advanced materials, read “Cobalt Alloys: Properties, Applications, and Challenges“. It outlines the mechanical properties, practical uses, and limitations of cobalt alloys in precision manufacturing and industrial applications.
Understanding the melting points of common metals is essential for safe, efficient, and accurate metalworking. Whether you are casting, welding, or fabricating, knowing how each material behaves under heat helps you achieve consistent results and maintain equipment performance. Keep this guide nearby whenever working on heat-based or temperature-sensitive metal projects.











