Why Crystal Structure Matters in Metal Performance
A metal’s response to heat, stress, and manufacturing operations is strongly influenced by its crystal structure, which refers to the internal arrangement of atoms. Mechanical properties such as strength, ductility, machinability, and fracture behavior are shaped by how atoms are packed and how they move under load. Understanding crystal structure helps explain why certain metals bend easily, others resist deformation, and some require controlled temperature conditions during forming or cutting.
What Is a Crystal Structure?
Crystal structure describes the repeating, orderly arrangement of atoms within a solid metal. Rather than being randomly positioned, atoms form predictable lattice patterns that influence how the material reacts to stress and temperature changes. In fabrication and engineering applications, three metallic crystal structures are most commonly encountered:
- Face-Centered Cubic (FCC)
- Body-Centered Cubic (BCC)
- Hexagonal Close-Packed (HCP)
Each structure affects how atoms shift relative to one another during deformation.
Slip Systems and Plastic Deformation
Plastic deformation occurs when atoms move along defined planes and directions within the crystal lattice, known as slip systems. The number and orientation of these slip systems determine how easily a metal can deform without cracking. Metals with more available slip systems typically allow smoother deformation, while those with fewer slip systems may show limited formability or temperature-dependent behavior.
Face-Centered Cubic (FCC) Metals
FCC metals have atoms located at each corner of the cube and at the center of each face. This arrangement provides multiple closely packed slip planes, allowing atoms to move efficiently under applied stress. As a result, FCC metals are generally easy to form at room temperature and show stable mechanical behavior across a wide temperature range. These characteristics support bending, shaping, and deep drawing operations.
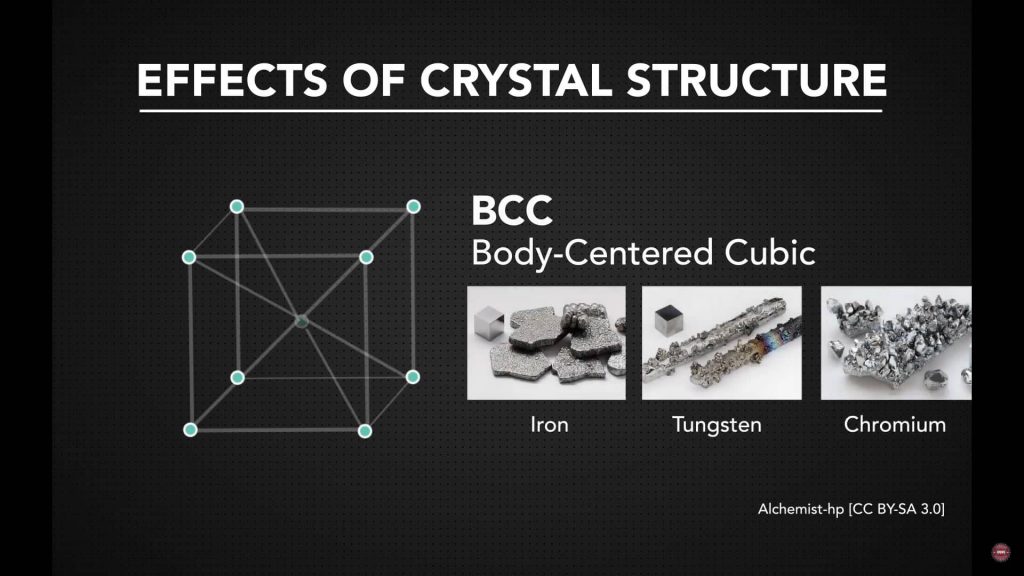
Body-Centered Cubic (BCC) Metals
BCC metals contain atoms at the corners of the cube and one atom at the center. While BCC structures also have multiple slip systems, these planes are not as closely packed as those in FCC metals. This reduces atomic mobility at lower temperatures, making BCC metals less accommodating during cold forming. As temperature increases, slip activity improves, which is why hot forming is often preferred for BCC materials.
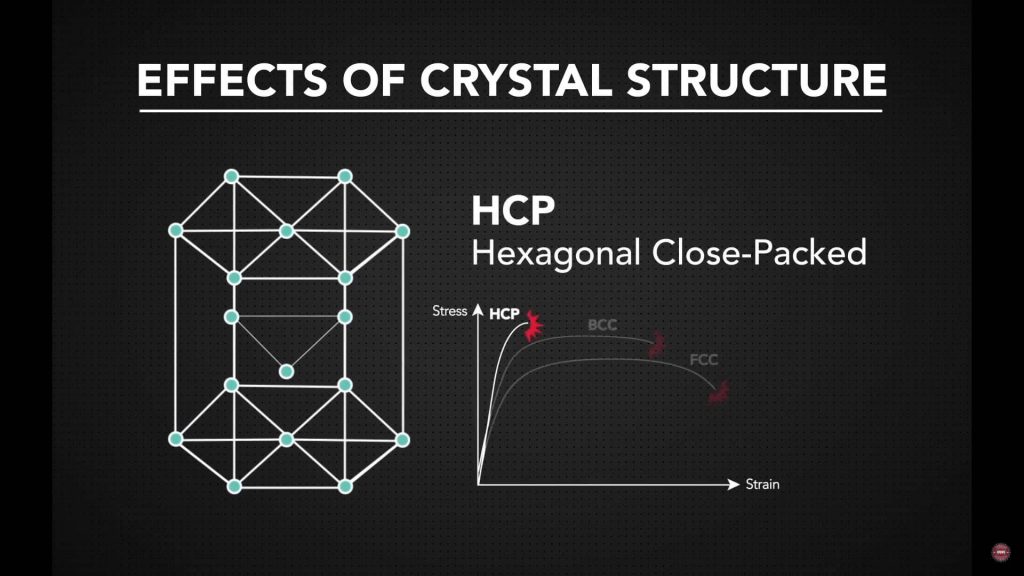
Hexagonal Close-Packed (HCP) Metals
HCP metals feature a tightly packed hexagonal atomic arrangement with fewer available slip systems. This limited atomic movement can restrict deformation at room temperature, particularly along certain directions. However, this does not imply reduced strength. Instead, it indicates that forming performance depends heavily on temperature and loading direction. Controlled forming conditions are commonly required to achieve consistent results.
Crystal Structure Comparison Table
| Crystal Structure | Slip System Availability | Ductility Trend | Temperature Sensitivity | Common Metals | Manufacturing Implications |
|---|---|---|---|---|---|
| FCC | High | High at room temperature | Low | Aluminum, Copper, Nickel | Easy forming, stable weld behavior |
| BCC | Moderate | Temperature dependent | High | Carbon steel, Chromium | Limited cold forming, hot forming preferred |
| HCP | Limited | Directionally dependent | Moderate | Magnesium, Titanium | Controlled forming required |
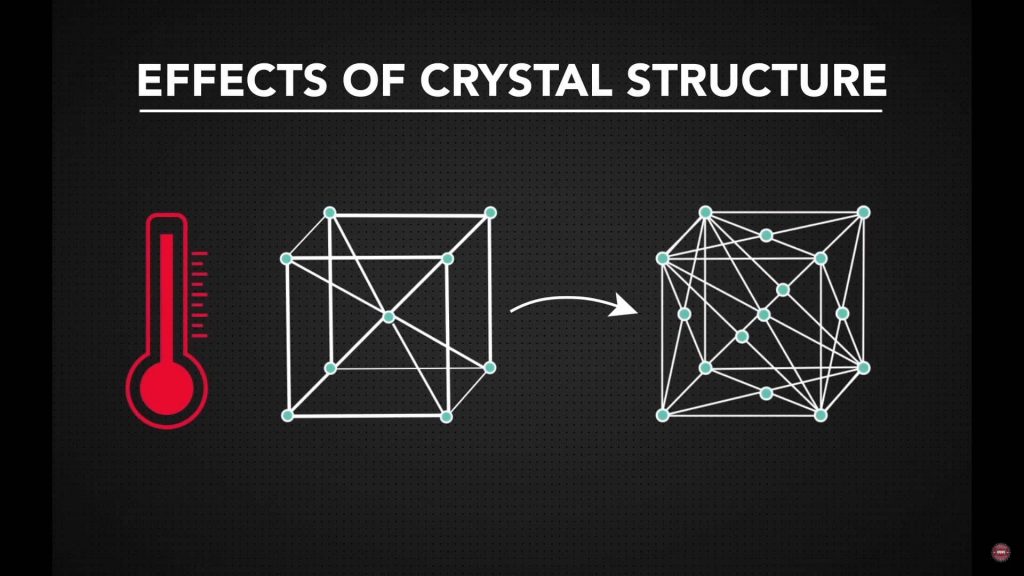
Temperature Effects on Crystal Structure
Some metals experience changes in crystal structure as temperature increases. Iron is a well-known example, transitioning from a BCC structure at ambient temperature to an FCC structure at elevated temperatures. This shift improves atomic mobility, reduces resistance during deformation, and supports hot forming operations. Recognizing these transitions helps explain why certain processes are performed at higher temperatures.
Test Your Knowledge on Crystal Structure Effects
Understanding how crystal structure influences metal behavior is easier when you can apply the concepts in practice. To reinforce what you’ve learned, visit sawbladeuniversity.com and take our quiz focused on the effects of crystal structure on metal properties. It’s a practical way to review key concepts, check your understanding, and connect theory with real manufacturing and machining scenarios.
Manufacturing Implications of Crystal Structure
Crystal structure influences multiple aspects of manufacturing performance, including:
- Formability during bending and shaping
- Cutting force and chip formation
- Tool wear during machining
- Weld behavior and heat-affected zone response
Selecting materials with suitable crystal structures helps maintain process stability and consistent part quality.
Crystal Structure and Industrial Use Cases
Material selection across industries often reflects crystal structure behavior:
- Aerospace: Titanium alloys (HCP) selected for strength-to-weight balance with controlled forming
- Construction: Carbon steels (BCC) used for structural and load-bearing roles
- Automotive: Aluminum alloys (FCC) chosen for lightweight forming and energy absorption
These applications rely on predictable responses to stress and temperature.
Learn More About Working With Magnesium Alloys
Magnesium alloys present unique handling and processing considerations due to their crystal structure, temperature sensitivity, and reactivity during cutting and forming. If you want a clearer understanding of how these materials behave in real manufacturing conditions, the article “Working with Magnesium Alloys: Tips and Techniques” offers practical insights into material preparation, machining practices, and process control. It’s a useful follow-up for readers looking to apply crystal structure knowledge to magnesium-specific applications.
How Crystal Structure Influences Failure Behavior
Fracture behavior is closely tied to crystal structure. FCC metals often deform noticeably before failure, providing visible warning signs. BCC metals may show brittle characteristics at lower temperatures but become more tolerant as temperature rises. HCP metals can fracture if deformation exceeds preferred directions. Understanding these tendencies improves safety planning and design decisions.
Crystal structure plays a central role in how metals behave during cutting, forming, welding, and service use. By understanding how FCC, BCC, and HCP structures respond to stress and temperature, manufacturers and operators can make informed choices about material selection, processing methods, and expected performance. Atomic arrangement directly influences real manufacturing results and should be considered a practical design factor rather than a theoretical concept.











