Why Crystal Structure Influences Metal Behavior
A metal’s ability to stretch, bend, or resist force is closely linked to its internal arrangement. The way atoms are positioned inside the material determines how easily they shift when stress is applied. Some structures restrict motion, while others allow movement without failure. Among common crystal types, the FCC arrangement is known for allowing extensive slip, which increases ductility and overall formability.
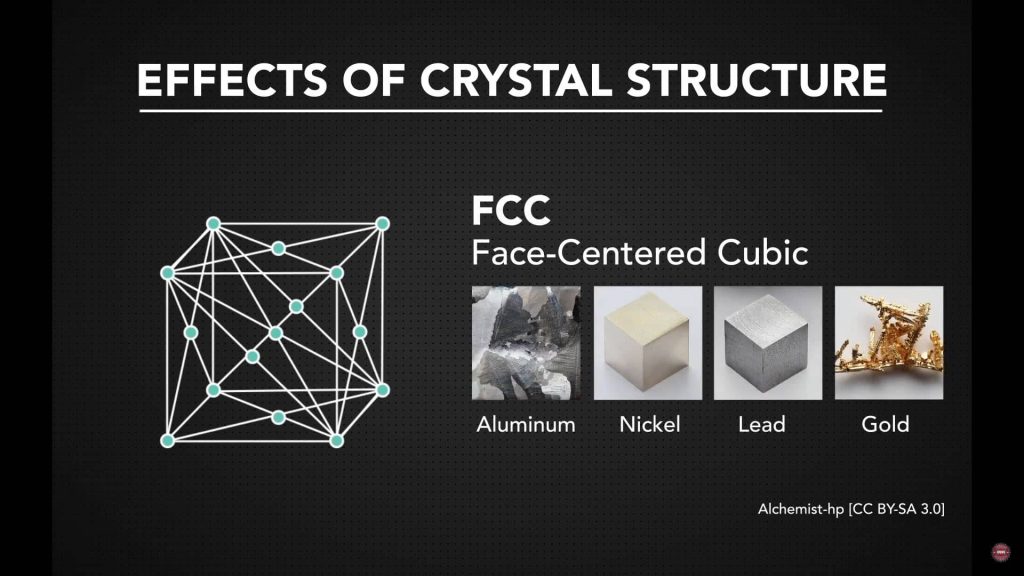
Key Features of FCC Crystal Structure
• Supports multiple slip planes
• Allows atomic layers to move with less resistance
• Performs well when stretched
• Maintains ductility at lower temperatures
• Suitable for shaping and forming methods
• Common in metals that require regular bending or reduction
• Performs reliably under repeated loading
How FCC Structure Supports High Ductility
In FCC metals, atoms are positioned in a way that allows layers to slide past one another while staying intact. This movement prevents sudden fracture and enables the metal to stretch under load. Because FCC materials provide numerous active slip systems, they work well in bending, rolling, forming, and other shaping processes.
FCC vs HCP and BCC Structures
Different crystal structures influence how a metal responds to force. HCP structures offer limited atomic mobility, which reduces ductility. BCC structures include more slip systems, but many activate only at higher temperatures. FCC metals retain slip activity across a broad temperature range, making them easier to form under common working conditions.

Crystal Structure Comparison Table
| Structure Type | Example Metals | Ductility Level | Slip System Behavior |
|---|---|---|---|
| FCC | Aluminum, Nickel, Gold, Lead | High | Active across a wide temperature range |
| BCC | Iron, Tungsten, Chromium | Moderate | Requires higher temperatures for improved slip |
| HCP | Titanium, Magnesium | Low | Few slip planes active at room temperature |
Temperature Changes and Structural Transformation
Some metals shift from one structure to another as temperature changes. Added heat modifies internal energy, allowing atoms to rearrange. Iron is a good example: it changes from BCC at room temperature to FCC at elevated temperatures. This higher-temperature form supports better shaping and metalworking.
Test Your Knowledge on Crystal Structures
If you want to check how much you’ve learned about FCC, BCC, and HCP metals, you can take our quick quiz on SawbladeUniversity.com. It’s a simple way to review the main ideas behind crystal structures, ductility, and deformation behavior. The quiz gives instant feedback and helps reinforce the concepts that matter most when working with metals in engineering or machining.
Advantages of FCC Metals in Manufacturing
• Excellent response to shaping and bending
• Resist cracking during forming
• Perform well in high-speed production
• Useful in applications requiring flexibility
• Stable during processes involving repeated deformation
Common FCC Metals and Their Uses
Aluminum’s ability to bend without cracking makes it ideal for transportation components. Nickel is used when strength and durability are needed. Gold and lead rely on their ductility for wiring, shaping, or forming. Each of these metals benefits from the FCC arrangement that supports smooth deformation.
Slip Systems and Deformation Behavior
FCC metals feature slip systems that stay active during standard forming conditions. Because they operate at room temperature, shaping operations become predictable and dependable. This lowers the risk of sudden failure and supports consistent performance in industries that rely on bending or stretching metal.
Why FCC Metals Remain Important in Engineering
FCC metals are used throughout construction, automotive manufacturing, electrical systems, and structural applications. Their ductility and stability make them suitable for parts that must absorb force, adjust under load, or pass through shaping processes before assembly.

Explore More on Metal Composition
If you want a clearer understanding of how different elements change the strength, hardness, and behavior of metals, take a moment to read “Steel Alloys: What’s in the Mix and Why It Matters.” It explains how alloying elements influence performance and why certain blends are chosen for tools, structures, and precision components. This added perspective pairs well with what you’ve learned here and helps connect crystal structure with real-world material selection.
Practical Benefits of FCC Metals
• Smooth deformation during forming
• Lower risk of cracking
• Reliable behavior under stress
• Good response to low-temperature conditions
• Suitable for thin sheets, wire, and shaped components
The FCC structure plays a major role in how metals respond to force. Materials such as aluminum, nickel, and gold benefit from the arrangement of atoms that supports movement across many slip systems. Understanding how FCC differs from HCP and BCC helps explain why some metals bend easily while others remain stiff. This knowledge supports better decisions in metal selection, shaping operations, and engineering design.











